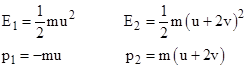|
Frequencies and Photons |
|
|
|
To measure the speed of an approaching (reflective) object, one method is to emit an electromagnetic wave of a given frequency and measure the frequency of the reflected wave. In terms of inertial coordinates in which the transmitter is at rest, let t1 and t2 denote the times of emission of two successive wave crests, and let t3 and t4 denote the times when the reflected wave crests arrive back at the emitter. Since the wave crests propagate at speed c in both directions, the distances to the wave crest reflection points are D1 = (t3 – t1)c/2 and D2 = (t4 – t2)c/2, and the times of the reflection events are T1 = (t1 + t3)/2 and T2 = (t2 + t4)/2. Therefore, the speed of the object (positive in the direction of the emitter) is |
|
|
|
|
|
|
|
The frequencies are inversely proportional to the time intervals between successive wave crests, so this gives |
|
|
|
|
|
|
|
This is the exact relativistic expression for the reflected Doppler effect. In many applications the change in velocity is extremely small, so we have the familiar approximation 2(v/c) = Δν/ν. Note that this result is not changed if there is a fixed delay between incident wave crests striking the object and the reflected wave crests departing the object. The derivation depends only on the premise that the wave propagates at c while in transit. |
|
|
|
Despite the clarity and simplicity of this derivation, some might object to it on the grounds that it is expressed in terms of classical electromagnetic waves, rather than the photons of quantum electrodynamics. This objection is somewhat misguided, because if we could precisely control the emissions and reflections of individual photons, we could simply repeat the above derivation for two consecutive photons instead of wave crests, and arrive at exactly the same result. However, this is based on regarding the “frequency” of the signal as the rate of emitting individual photons, which is a different frequency from what is often called “the frequency of the photon”. We place this phrase in quotes, because what is sometimes loosely referred to as the frequency of a photon is actually the frequency of the source. A photon is better described as an interaction, and (assuming light is massless) it carries a single phase, which does not advance during the transit. |
|
|
|
For example, we can cause a charged particle to move back and forth along a line segment, creating a cyclical motion with a certain frequency, resulting in the emission of electromagnetic radiation, and the emitted photons comprising this radiation each have an amount of energy proportional to that frequency. Now, a photon is not a classical particle, i.e., it does not have a trajectory through space. It is an excitation of the quantum field governed by the relativistic Schrodinger wave equation. A wavy diffraction pattern arises in a two-slit experiment because the probability of a photon landing in any particular spot is equal to the magnitude of the sum of the phased amplitudes for each possible path that is open to the photon. The combination of phases, advancing at a rate determined by the frequency of the source, yields the wavelike behavior of photons. We sometimes think of a photon having a frequency, even though it really just carries a single phase, because the probability amplitudes are composed of the phases for trajectories that originate at different points in the cycle of the source, which is why the wave pattern depends on the frequency of the source. |
|
|
|
The frequency (rate) of emission of photons is actually a measure of the intensity of the radiation, whereas the frequency of the source (which is commonly termed ‘the frequency of the photon’, even though that is somewhat misleading) determines energy of the individual photon interactions. But both of these can be represented as the frequency of a time-varying signal, so they are both affected in exactly the same proportion by the Doppler effect. Also, we know from special relativity that energy too transforms in proportion to frequency, so we are assured that the energy of individual photons transforms in the same way as the frequency of the associated wave. This is why we can detect the Doppler shift for a single photon by measuring it’s energy (assuming it has the wavelength to be perfectly reflected rather than absorbed). |
|
|
|
To examine this in detail, it’s worthwhile to begin by considering a speed measurement performed with a classical particle. In the context of Newtonian mechanics, suppose an object is approaching us at some speed v, and we propel a small elastic particle of mass m toward the approaching object with speed u. |
|
|
|
|
|
|
|
Assuming a perfectly elastic collision, and assuming the mass of the approaching object is very much greater than the mass of the particle (so the object’s speed is essentially unaffected), the returning particle will have speed u + 2v. Thus the difference between the speeds of the particle when it is emitted and when it returns is 2v, so we can remotely determine the speed v. But what about the energy of the particle? |
|
|
|
The kinetic energies and momenta of the particle at emission and at reception are |
|
|
|
|
|
|
|
(The momenta have opposite signs because they are in opposite directions, and we take positive in the approaching direction.) Hence the changes in these quantities are |
|
|
|
|
|
|
|
so we have |
|
|
|
|
|
This is actually a more general result, since it applies in relativistic mechanics as well, shown by integrating Fds and Fdt, but this doesn’t really tell us anything new, since it’s easier to just note that v is half the difference between emission and reception speeds. However, suppose we replace our massive elastic particle with a ‘photon’, and import some facts about photons from quantum theory, such as the relations E = hν = cp where ν signifies “the frequency of the photon” (noting the caveats above). This implies the change in frequency is proportional to the change in energy, i.e., we have ΔE = hΔν. Also, the momentum of the particle at emission is –hν1/c, and at reception is h(ν1 + Δν)/c, so the change in momentum is Δp = h(2ν1 + Δν)/c. Thus from the above equation we have |
|
|
|
|
|
|
|
The second term in the denominator is negligible for low velocities, so we again have the familiar approximate result 2(v/c) = Δν/ν. |
|
|
|
Needless to say, the fact we get the same result for photon frequencies based on energy and momentum as we got from the simple kinematics of wave frequencies is not some magical coincidence. The frequency of a photon is really the frequency of the source, just as in the emission of classical waves, so the same kinematic analysis applies. In both cases we are just projecting the proper time of the source onto the target (and back) along null intervals. This is obscured in the analysis based on photon energy and momentum by smuggling the quantum relations, which implicitly entail the relativistic kinematics. So the photon analysis doesn’t add anything to our understanding of the Doppler effect – in fact, it obscures the understanding, and can’t actually be justified on its own terms, because the reflection of a single photon is a problematic concept. If a photon from a monochromatic source strikes an object, it may or may not be reflected (specular scattering versus being absorbed thermally or by elevating an electron orbital state and possibly being re-emitted) at the surface, depending on whether it encounters an atom with an electron that can be elevated to an energy state of the compatible level. Also, an individual photon interacts by the transference of energy, so the “frequency of a single photon” is not directly measurable, other than by measuring the energy and inferring the frequency. At best, analyses of the Doppler effect in terms of a single photon merely demonstrate consistency between the imported quantum relations and the relativistic dynamics. |
|
|